More about the Chemistry
Home » Intro to OA »
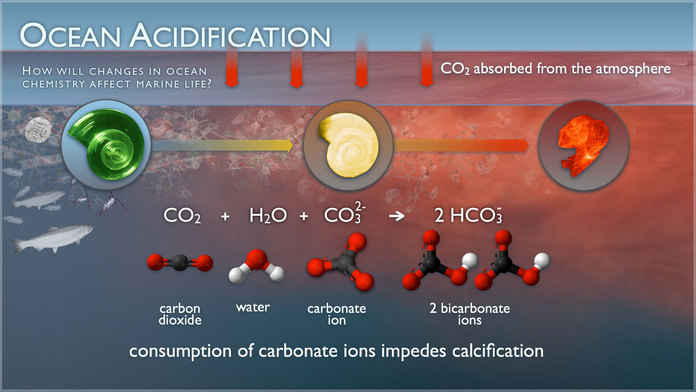
Increases in carbon dioxide (CO2) in the atmosphere drive corresponding increases in dissolved CO2 within the surface waters of our ocean. This dissolved CO2, sometimes referred to as an “acid gas,” reacts with seawater to form carbonic acid (H2CO3). Carbonic acid almost completely dissociates to form bicarbonate ions (HCO3–) and hydrogen ions (H+). The increase in the concentration of hydrogen ions (H+) from these reactions causes the seawater to become more acidic (and is what the “H” in “pH” represents), hence the term “ocean acidification” and link to pH. However, seawater is naturally buffered to resist large changes in pH by reacting some of this excess H+ with carbonate ions (CO32-). Additionally, this increase in hydrogen ions (H+) favors the formation of bicarbonate ions (HCO3–) over carbonate ions (CO32-), making carbonate ions relatively less abundant. These carbonate ions (CO32-) are an important part of calcium carbonate (CaCO3) structures, such as sea shells and coral skeletons. Decreases in seawater carbonate ions can make building and maintaining shells and other calcium carbonate structures difficult for calcifying marine organisms such as coral, plankton, and shellfish. From NOAA OAP »
What is ‘aragonite saturation’ and why is it important?
Aragonite is a form of calcium carbonate that organisms use to build their shells and skeletons and aragonite saturation tells us how much of this mineral is available to them in seawater. Ocean acidification lowers the concentration of this building block in the ocean and the more carbon dioxide dissolved in the ocean or the more runoff of freshwater from glacial melt, the lower the aragonite saturation state. As aragonite saturation goes down, it gets harder for shelled organisms and corals to build their shells and skeletons. Aragonite saturation is given the sympol Ωar. An aragonite saturation state of 1 is considered the tipping point, below which we might expect some shells and skeletons to dissolve. Alaskan waters approach this tipping point seasonally and may cross this threshold at some depths and locations within only a few decades. See an explanation with equations ».
